„Worst Case Scenario”: MacroGenics Crashes After Five Patient Deaths In Experimental Drug Trial
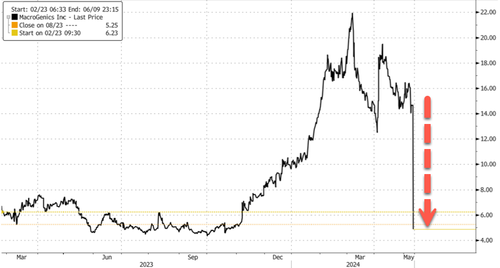
A Rockville, Maryland-based biotech company called MacroGenics crashed in premarket trading in New York after it revealed five deaths in a clinical trial of its investigative therapy for prostate cancer, according to Bloomberg.
MacroGenics finished enrolling participants for the TAMARACK Phase 2 study of vobra duo in November 2023. This study involves patients with metastatic castration-resistant prostate cancer who have previously received one treatment targeting the androgen receptor pathway. These participants might also have had one prior treatment, including taxane, but no other chemotherapy. The study aims to test two different doses of vobra duo, either 2.0 mg/kg or 2.7 mg/kg, given every four weeks.
„The interim safety and anti-tumor activity observed to date in the TAMARACK study look very promising for patients with metastatic castration-resistant prostate cancer,” Johann DeBono, Regius Professor of Cancer Research and Professor in Experimental Cancer Medicine at The Institute of Cancer Research, London and The Royal Marsden NHS Foundation Trust, stated in a release.
DeBono continued, „With the limited treatment options currently available to these patients, this novel ADC molecule could potentially become the first therapy targeting B7-H3 in patients with prostate cancer and would represent an important new treatment for this population.”
Further in the release, MacroGenics revealed five deaths in the study. They said two deaths have been considered unrelated to the study, while the other three are being investigated.
A total of five events with fatal outcome occurred as follows: one Grade 5 event in the 2.0 mg/kg dosing cohort: acute myocardial infarction (considered unrelated to study drug by the investigator); three Grade 5 events in the 2.7 mg/kg dosing cohort: one • cardiac arrest (considered unrelated to study drug by the investigator) and two events of pneumonitis. In addition, a patient in the 2.7 mg/kg dosing cohort had a Grade 3 pleural effusion that is recorded as having a fatal outcome. The latter three deaths are being investigated, as follow-up is incomplete on this ongoing trial.
In premarket trading, shares crashed 67% on fears of updated efficacy and safety information regarding the clinical trial.
Wall Street analysts were very sour about the new developments. Here’s what they’re saying (list courtesy of Bloomberg):
BMO Capital Markets (downgrades to market perform from outperform)
- Analyst Etzer Darout has lower conviction on MacroGenics’ prostate cancer program, with both efficacy and safety updates for TAMARACK falling short of expectations
Stifel (downgrades to buy from hold)
Analyst Stephen Willey’s primary concerns are not efficacy- driven, but rather reflect safety and tolerability data
The data doesn’t appear meaningfully differentiated from the prior P1 dose-expansion experience
SVB Securities (outperform)
- TAMARACK data was notable for its meaningfully deteriorated safety profile which „likely represent one of the worst case scenarios,” says analyst Jonathan Chang
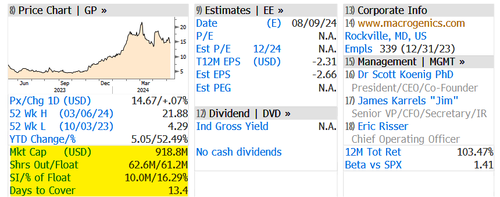
… bears shorted 16.29% of the float or 10 million shares.
This sets up for a high volume day and wild volatility.
Tyler Durden
Fri, 05/10/2024 – 12:10